ʻO ka maʻi maʻi nosocomial ka maʻi nosocomial maʻamau a koʻikoʻi, kahi o ka maʻi pneumonia pili i ka ventilator (VAP) he 40%. ʻO ka VAP i hoʻokumu ʻia e nā pathogens refractory he pilikia paʻakikī. No nā makahiki, ua ʻōlelo nā alakaʻi i nā ʻano hana (e like me ka sedation i hoʻopaʻa ʻia, ke kiʻekiʻe o ke poʻo) e pale ai i ka VAP, akā loaʻa ka VAP i ka 40% o nā maʻi me ka intubation tracheal, ka hopena o ka noho lōʻihi o ka haukapila, hoʻonui i ka hoʻohana ʻana i nā lāʻau antibiotic, a me ka make. Ke ʻimi mau nei ka poʻe i nā hana pale ʻoi aku ka maikaʻi.
ʻO ka maʻi pneumonia pili i ka Ventilator (VAP) kahi hoʻomaka hou o ka maʻi pneumonia e ulu ana he 48 mau hola ma hope o ka hoʻokomo ʻana i ka tracheal a ʻo ia ka maʻi nosocomial maʻamau a make i ka hale mālama kino (ICU). Ua hoʻokaʻawale ka American Society of Infectious Diseases Guidelines i ka VAP mai ka wehewehe ʻana o ka maʻi maʻi i loaʻa i ka haukapila (HAP) (e pili ana ka HAP i ka maʻi maʻi i loaʻa ma hope o ka hoʻokipa ʻana me ka ʻole o ka paipu tracheal a ʻaʻole pili i ka ea uila; ʻo VAP ka pneumonia ma hope o ka hoʻokomo ʻana o ka tracheal a me ka hoʻoheheʻe ʻana i ka mīkini), a ke manaʻoʻiʻo nei ka European Society a me Kina he ʻano HAP-1 kūikawā.
I nā poʻe maʻi e loaʻa ana i ka mechanical ventilation, ʻo ka nui o ka VAP mai ka 9% a hiki i ka 27%, ka nui o ka make ma 13%, a hiki iā ia ke alakaʻi i ka hoʻohana ʻana i ka antibiotic systemic, hoʻolōʻihi i ka mechanical ventilation, lōʻihi ka noho ʻana o ka ICU, a me nā kumukūʻai hoʻonui [4-6]. ʻO ka HAP/VAP i nā poʻe maʻi non-immunodeficient ke kumu maʻamau e ka maʻi bacteria, a ʻokoʻa ka puʻunaue ʻana o nā pathogens maʻamau a me ko lākou mau ʻano kū'ē i ka ʻāina, ka papa halemai, ka heluna kanaka maʻi, a me ka hoʻolaha ʻana i ka lāʻau antibiotic, a loli i ka manawa. Ua lanakila ʻo Pseudomonas aeruginosa i nā pathogens pili i ka VAP ma ʻEulopa a me ʻAmelika, ʻoiai ʻoi aku ka nui o Acinetobacter baumannii i hoʻokaʻawale ʻia i nā halemai kula kiʻekiʻe ma Kina. Hoʻokahi hapakolu a i ka hapalua o nā make pili i ka VAP e pili pono ana i ka maʻi, me ka nui o ka make o nā hihia i hoʻokumu ʻia e Pseudomonas aeruginosa a me acinetobacter ke kiʻekiʻe [7,8].
Ma muli o ka heterogeneity ikaika o VAP, he haʻahaʻa ka kikoʻī diagnostic o kāna mau hōʻike lapaʻau, kiʻi kiʻi a me nā hoʻokolohua hoʻokolohua, a he ākea ka laulā o ka maʻi ʻokoʻa, kahi mea paʻakikī ke ʻike i ka VAP i ka manawa. I ka manawa like, he paʻakikī koʻikoʻi ka pale bacteria i ka mālama ʻana i ka VAP. Ua manaʻo ʻia ʻo ka pilikia o ka hoʻomohala ʻana i ka VAP he 3%/lā i nā lā 5 mua o ka hoʻohana ʻana i ka ea mechanical, 2%/lā ma waena o 5 a me 10 mau lā, a me 1%/lā no ke koena o ka manawa. Loaʻa ka hopena kiʻekiʻe ma hope o 7 mau lā o ka hoʻoheheʻe ʻana, no laila aia kahi puka e hiki ai ke pale ʻia ka maʻi ma mua [9,10]. Ua nānā nā haʻawina he nui i ka pale ʻana i ka VAP, akā ʻoiai he mau makahiki o ka noiʻi ʻana a me ka hoʻāʻo ʻana e pale i ka VAP (e like me ka pale ʻana i ka intubation, ka pale ʻana i ka intubation, hoʻemi i ka hoʻohaʻahaʻa, hoʻokiʻekiʻe i ke poʻo o ka moena e 30 ° a 45 °, a me ka mālama waha), ʻaʻole ʻike ʻia ka hōʻemi ʻana a ʻoi aku ka kiʻekiʻe o ke kaumaha olakino pili.
Ua hoʻohana ʻia nā lāʻau antibiotic inhaled e mālama i nā maʻi maʻi ea mai ka makahiki 1940. No ka mea hiki iā ia ke hoʻonui i ka lawe ʻana i nā lāʻau lapaʻau i ka wahi i manaʻo ʻia o ka maʻi (ʻo ia hoʻi ke ea) a hōʻemi i nā hopena ʻaoʻao ʻōnaehana, ua hōʻike ʻo ia i ka waiwai hoʻohana maikaʻi i nā maʻi like ʻole. Ua ʻae ʻia nā antibiotic inhaled e ka US Food and Drug Administration (FDA) a me ka European Medicines Agency (EMA) no ka hoʻohana ʻana i ka cystic fibrosis. Hiki i nā lāʻau antibiotic inhaled ke hoʻemi nui i ka ukana bacteria a me ka pinepine o ka exacerbations i ka bronchiectasis me ka hoʻonui ʻole ʻana i nā hanana pōʻino āpau, a ua ʻike nā alakaʻi o kēia manawa i ka lāʻau lapaʻau mua no nā maʻi me ka maʻi pseudomonas aeruginosa a me nā exacerbations pinepine; Hiki ke hoʻohana ʻia nā lāʻau lapaʻau inhaled i ka wā perioperative o ka hoʻololi ʻana i ka māmā e like me nā lāʻau adjuvant a prophylactic [11,12]. Akā, i ka 2016 US VAP alakaʻi, poʻe akamai i nele i ka hilinaʻi i ka pono o ka adjuvant inhaled antibiotic ma muli o ka nele o ka nui randomized mana ho'āʻo. ʻAʻole i loaʻa nā hopena maikaʻi i ka Phase 3 (INHALE) i paʻi ʻia i ka makahiki 2020 (inhale amikacin i kōkua i nā lāʻau antibiotic intravenous no ka maʻi bacterial Gram-negative i hoʻokumu ʻia e nā maʻi VAP, kahi makapō ʻelua, randomized, placebos controlled, phase 3 efficacy trial, he 807 mau mea maʻi, lāʻau ʻōnaehana + kōkua inhalation o 10 mau lā).
Ma kēia pōʻaiapili, ua hana kekahi hui i alakaʻi ʻia e nā mea noiʻi mai ka Regional University Hospital Center of Tours (CHRU) ma Farani i kahi hoʻolālā noiʻi ʻokoʻa a alakaʻi i kahi hoʻokolohua hoʻāʻo i hoʻomaka ʻia e ka mea noiʻi, multicenter, double-blind, randomized controlled efficacy trial (AMIKINHAL). Ua hoʻohālikelike ʻia ka amikacin inhaled a i ʻole placebo no ka pale ʻana i ka VAP ma 19 icus ma Farani [13].
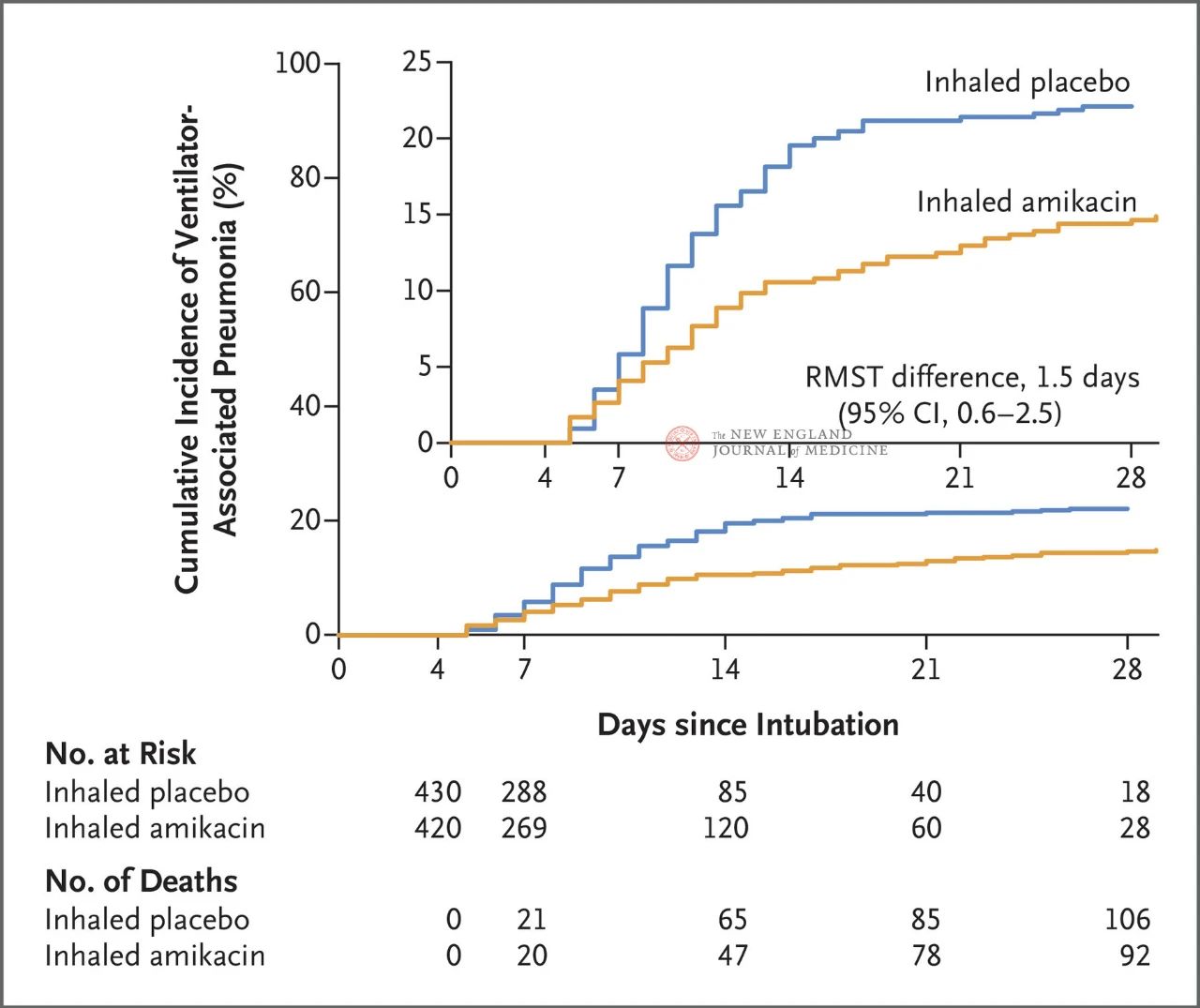
ʻO ka huina o 847 mau maʻi makua me ka invasive mechanical ventilation ma waena o 72 a me 96 hola i hāʻawi ʻia i ka 1: 1 i ka inhalation o ka amikacin (N = 417,20 mg / kg ke kaumaha kino kūpono, QD) a i ʻole ka inhalation o kahi placebo (N = 430, 0.9% sodium chloride like) no 3 mau lā. ʻO ka hopena mua ka māhele mua o ka VAP mai ka hoʻomaka ʻana o ka hana i koho ʻia a hiki i ka lā 28.
Ua hōʻike ʻia nā hopena o ka hoʻokolokolo ma 28 mau lā, 62 mau maʻi (15%) i ka hui amikacin i hoʻomohala i ka VAP a me 95 nā mea maʻi (22%) i ka hui placebo i hoʻomohala i ka VAP (ʻo ka palena ʻokoʻa o ke ola ʻana no VAP he 1.5 lā; 95% CI, 0.6~2.5; P=0.004).
Ma ke ʻano o ka palekana, ʻehiku mau maʻi (1.7%) ma ka hui amikacin a me ʻehā mau maʻi (0.9%) ma ka hui placebo i loaʻa i nā hanana koʻikoʻi pili i ka hoʻokolokolo. Ma waena o ka poʻe i loaʻa ʻole i ka hōʻeha ʻeha ma ka randomization, 11 mau mea maʻi (4%) i ka hui amikacin a me 24 mau mea maʻi (8%) i ka hui placebo i hōʻeha ʻeha i ka lā 28 (HR, 0.47; 95% CI, 0.23 ~ 0.96).
ʻEkolu mau mea koʻikoʻi o ka hoʻokolohua lapaʻau. ʻO ka mea mua, ma ke ʻano o ka hoʻolālā haʻawina, hoʻāʻo ka AMIKINHAL i ka hoʻāʻo IASIS (kahi hoʻāʻo randomized, double-blind, placebo-controlled, parallel phase 2 hoʻokolohua e pili ana i nā maʻi 143). No ka loiloi i ka palekana a me ka pono o ka amikacin - fosfomycin inhalation systemic lapaʻau o gram-negative bacteria maʻi maʻi maʻi maʻi ma muli o VAP) a me ka INHALE ho'āʻo e hoʻopau me nā hopena maikaʻi ʻole nā haʻawina i aʻo ʻia, e kālele ana i ka pale ʻana i ka VAP, a loaʻa nā hopena maikaʻi. Ma muli o nā hiʻohiʻona o ka make nui a me ka noho lōʻihi o ka haukapila i nā mea maʻi me ka mechanical ventilation a me ka VAP, inā hiki i ka inhalation amikacin ke hoʻokō i nā hopena like ʻole i ka hōʻemi ʻana i ka make a me ka noho ʻana o ka haukapila i kēia mau maʻi, e ʻoi aku ka waiwai no ka hana lapaʻau. Eia nō naʻe, ma muli o ke ʻano like ʻole o ka mālama ʻana a me ka mālama ʻana i kēlā me kēia maʻi a me kēlā me kēia kikowaena, aia kekahi mau kumu hoʻohālikelike e hiki ke hoʻopilikia i ke aʻo ʻana, no laila paʻakikī paha ka loaʻa ʻana o kahi hopena maikaʻi e pili ana i nā lāʻau antibiotic inhaled. No laila, ʻaʻole pono ka hoʻolālā hoʻonaʻauao maikaʻi wale nō i ke aʻo ʻana i ka hoʻomaʻamaʻa maikaʻi, akā ke koho pū ʻana i nā hopena kumu kūpono.
ʻO ka lua, ʻoiai ʻaʻole ʻōlelo ʻia nā antibiotic aminoglycoside ma ke ʻano he lāʻau lapaʻau hoʻokahi i nā alakaʻi VAP like ʻole, hiki i nā antibiotic aminoglycoside ke uhi i nā pathogens maʻamau i nā maʻi VAP (me ka pseudomonas aeruginosa, acinetobacter, etc.), a ma muli o kā lākou palena absorption i loko o nā pūnana epithelial lung, kiʻekiʻe ka nānā ʻana ma ke kahua o ka maʻi, a me ka haʻahaʻa systemic systemic. Hoʻohana nui ʻia nā lāʻau antibiotic aminoglycoside i waena o nā lāʻau lapaʻau inhaled. Ua kūlike kēia pepa me ka manaʻo kikoʻī o ka nui o ka hopena o ka hoʻokele intratracheal o ka gentamicin i nā laʻana liʻiliʻi i paʻi ʻia ma mua, e hōʻike pū ana i ka hopena o nā lāʻau antibiotic aminoglycoside inhaled i ka pale ʻana i ka VAP. Pono e hoʻomaopopo ʻia ʻo ka hapa nui o nā mana placebo i koho ʻia i nā hoʻokolohua e pili ana i nā lāʻau antibiotic inhaled he saline maʻamau. Eia naʻe, e noʻonoʻo ana i ka inhalation atomized o ka paʻakai maʻamau ponoʻī hiki ke pāʻani i kekahi kuleana i ka diluting sputum a me ke kōkua ʻana i ka expectorant, hiki i ka saline maʻamau ke hoʻopilikia i ka loiloi o nā hopena haʻawina, pono e noʻonoʻo ʻia i loko o ke aʻo ʻana.
Eia kekahi, he mea nui ka hoʻololi kūloko o ka lāʻau HAP/VAP, e like me ka prophylaxis antibiotic. I ka manawa like, me ka nānā ʻole i ka lōʻihi o ka manawa intubation, ʻo ka ecology o ka ICU kūloko ka mea koʻikoʻi koʻikoʻi no ka maʻi me ka maʻi bacteria kūʻē i nā lāʻau lapaʻau. No laila, pono e nānā i ka ʻikepili microbiology o nā halemai kūloko e like me ka hiki, a ʻaʻole hiki ke kuhikuhi makapō i nā alakaʻi a i ʻole ka ʻike o nā haukapila tertiary. I ka manawa like, hoʻohui pinepine ʻia nā maʻi maʻi e koi ana i ka hoʻoheheʻe mechanical me nā maʻi lehulehu, a ma lalo o ka hana hui ʻana o nā kumu he nui e like me ke kūlana stress, aia paha kahi hanana o ka microbes intestinal crosstalk i ka māmā. ʻO ke kiʻekiʻe o ka heterogeneity o nā maʻi i hoʻokumu ʻia e ka superposition o loko a me waho e hoʻoholo ai he lōʻihi loa ka hoʻolaha ʻana o ke kauka nui o kēlā me kēia hana hou.
Ka manawa hoʻouna: Dec-02-2023