Ua lilo ka chimeric antigen receptor (CAR) T cell therapy i mea lapaʻau koʻikoʻi no nā maʻi maʻi hematological hou a i ʻole refractory. I kēia manawa, ʻeono mau huahana auto-CAR T i ʻae ʻia no ka mākeke ma ʻAmelika Hui Pū ʻIa, ʻoiai ʻehā mau huahana CAR-T i helu ʻia ma Kina. Eia kekahi, ke kūkulu ʻia nei nā ʻano huahana autologous a allogeneic CAR-T. Ke hana nei nā hui lāʻau lapaʻau me kēia mau hanauna hou aku e hoʻomaikaʻi i ka maikaʻi a me ka palekana o nā lāʻau lapaʻau i loaʻa no nā maʻi maʻi hematological ʻoiai ke kuhikuhi nei i nā maʻi koko paʻa. Ke kūkulu ʻia nei nā pūnaewele CAR T e mālama i nā maʻi ʻino ʻole e like me nā maʻi autoimmune.
He kiʻekiʻe ke kumukūʻai o CAR T (i kēia manawa, ʻo ke kumukūʻai o CAR T/ CAR ma ʻAmelika Hui Pū ʻIa ma waena o 370,000 a me 530,000 US kālā, a ʻo nā huahana CAR-T ʻoi loa ma Kina he 999,000 yuan/kaʻa). Eia kekahi, ua lilo ke kiʻekiʻe o nā hopena ʻawaʻawa koʻikoʻi (ʻoi aku ka maʻi 3/4 immunoeffector cell-related neurotoxic syndrome [ICANS] a me ka cytokine release syndrome [CRS]) i mea keakea nui no ka poʻe haʻahaʻa a me ka loaʻa kālā waena e loaʻa iā CAR T cell therapy.
I kēia mau lā, ʻo ka Indian Institute of Technology Mumbai a me Mumbai Tata Memorial Hospital i ka launa pū ʻana e hoʻomohala i kahi huahana CD19 CAR T hou humanized (NexCAR19), ua like kona pono me nā huahana i loaʻa, akā ʻoi aku ka maikaʻi o ka palekana, ʻo ka mea nui ke kumukūʻai ʻo ka hapaʻumi wale nō o nā huahana like ʻo ʻAmelika Hui Pū ʻIa.
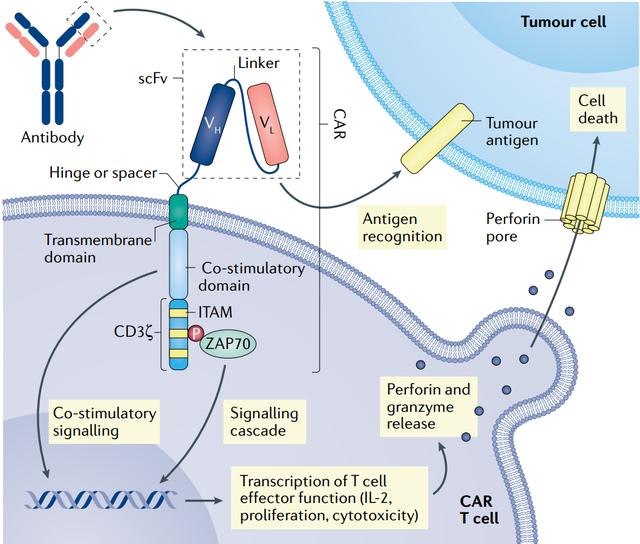
E like me ʻehā o nā lāʻau lapaʻau CAR T ʻeono i ʻāpono ʻia e ka US Food and Drug Administration (FDA), ʻo NexCAR19 pū kekahi e hoʻopaʻa i ka CD19. Eia nō naʻe, i nā huahana i ʻae ʻia ma ʻAmelika Hui Pū ʻIa, ʻo ka ʻāpana antibody ma ka hopena o ka CAR maʻamau e hele mai mai nā ʻiole, e kaupalena ana i kona hoʻomau ʻana no ka mea ʻike ʻia e ka ʻōnaehana pale ʻana he haole a hoʻomaʻemaʻe loa ia. Hoʻohui ʻo NexCAR19 i kahi protein kanaka i ka hopena o ka antibody ʻiole.
Ua hōʻike ʻia nā noiʻi Laboratory e hoʻohālikelike ʻia ka hana antitumor o "humanized" Cars me nā kaʻa i loaʻa i ka murine, akā me nā pae haʻahaʻa o ka hana cytokine i hoʻokomo ʻia. ʻO ka hopena, ʻoi aku ka liʻiliʻi o ka poʻe maʻi i ka hoʻomohala ʻana i ka CRS koʻikoʻi ma hope o ka loaʻa ʻana o CAR T therapy, ʻo ia ka mea e hoʻomaikaʻi ʻia ka palekana.
No ka hoʻohaʻahaʻa ʻana i nā kumukūʻai, ua kūkulu, hoʻāʻo a hana ka hui noiʻi ʻo NexCAR19 i ka huahana holoʻokoʻa ma India, kahi i ʻoi aku ka liʻiliʻi o ka hana ma mua o nā ʻāina loaʻa kālā.
No ka hoʻokomo ʻana i ka CAR i loko o nā pūnaewele T, hoʻohana maʻamau ka poʻe noiʻi i nā lentiviruses, akā he pipiʻi nā lentivirus. Ma ʻAmelika Hui Pū ʻIa, ʻo ke kūʻai ʻana i nā vector lentiviral lawa no kahi hoʻokolokolo 50-kanaka hiki ke kūʻai ʻia he $800,000. Ua hana nā kānaka ʻepekema ma ka ʻoihana hoʻomohala ʻo NexCAR19 i ka kaʻa lawe ʻana iā lākou iho, e hōʻemi nui ana i nā kumukūʻai. Eia kekahi, ua loaʻa i ka hui noiʻi India kahi ala ʻoi aku ka maikaʻi o ka hana nui ʻana i nā cell engineered, e pale ana i ka hoʻohana ʻana i nā mīkini automated pipiʻi. Ke kūʻai nei ka NexCAR19 i kēia manawa ma kahi o $48,000 no kēlā me kēia ʻāpana, a i ʻole he hapaʻumi o ke kumukūʻai o kāna hoa US. Wahi a ke poʻo o ka hui nāna i hoʻomohala iā NexCAR19, ke manaʻo nei e hoʻemi ʻia ke kumukūʻai o ka huahana i ka wā e hiki mai ana.
ʻO ka mea hope loa, ʻo ka hoʻomaikaʻi ʻana i ka palekana o kēia lapaʻau i hoʻohālikelike ʻia me nā huahana ʻē aʻe i ʻae ʻia e FDA, ʻo ia hoʻi, ʻaʻole pono ka hapa nui o nā maʻi e hoʻōla i loko o ka hale mālama koʻikoʻi ma hope o ka loaʻa ʻana o ka lāʻau lapaʻau, e hōʻemi hou ana i nā kumukūʻai no nā maʻi.
Ua hōʻike ʻo Hasmukh Jain, he kauka lapaʻau oncologist ma Tata Memorial Center ma Mumbai, i kahi hōʻuluʻulu ʻikepili hui o ka Phase 1 a me ka Phase 2 hoʻāʻo o NexCAR19 ma ka American Society of Hematology (ASH) 2023 hālāwai makahiki.
ʻO ka Phase 1 hoʻāʻo (n=10) he hoʻokolohua hoʻokahi-waena i hoʻolālā ʻia e hoʻāʻo i ka palekana o 1 × 107 a 5 × 109 CAR T cell doses i nā maʻi me ka relapsed/refractory diffuse nui B-cell lymphoma (r/r DLBCL), transforming follicular lymphoma (tFL), a me ka mediastinal nui B-cell lymphoma (PMBCL). ʻO ka hoʻāʻo ʻana o ka Phase 2 (n=50) he haʻawina hoʻokahi lima, multicenter i hoʻopaʻa inoa i nā mea maʻi ≥15 mau makahiki me nā maʻi maʻi r/r B-cell, e komo pū ana me nā lymphoma B-cell hoʻopaʻapaʻa a me ka leukemia lymphoblastic acute. Hāʻawi ʻia nā mea maʻi iā NexCAR19 ʻelua mau lā ma hope o ka loaʻa ʻana o ka fludarabine me ka cyclophosphamide. ʻO ≥5 × 107/kg CAR T ka mea i manaʻo ʻia. ʻO ka hopena mua, ʻo ia ka helu pane pane ʻana (ORR), a ʻo nā ʻaoʻao hope loa e pili ana i ka lōʻihi o ka pane, nā hanana ʻino, ke ola holomua ʻole (PFS), a me ke ola holoʻokoʻa (OS).
He 47 mau mea maʻi i mālama ʻia me NexCAR19, 43 o lākou i loaʻa i ka maʻi i hoʻopaʻa ʻia. He 33/43 (78%) nā mea maʻi i hoʻopau i ka loiloi 28-lā ma hope o ka infusion. ʻO ka ORR he 70% (23/33), a he 58% (19/33) i loaʻa i ka pane piha (CR). I loko o ka lymphoma cohort, ʻo ORR he 71% (17/24) a ʻo CR he 54% (13/24). I loko o ka maʻi leukemia cohort, ʻo ka helu CR he 66% (6/9, MRD-negative i 5 mau hihia). ʻO ka manawa hahai ma waena o nā maʻi loiloi he 57 mau lā (21 a 453 mau lā). Ma ka 3 - a me ka 12 mahina ka hahai ʻana, ʻeiwa mau maʻi a me ʻekolu hapaha o nā maʻi i mālama i ke kala.
ʻAʻohe make pili i ka mālama ʻana. ʻAʻohe o nā maʻi i loaʻa kahi pae o ICANS. 22/33 (66%) maʻi i hoʻomohala i ka CRS (61% papa 1/2 a me 6% papa 3/4). ʻO ka mea nui, ʻaʻohe CRS ma luna o ka papa 3 i loaʻa i ka cohort lymphoma. Loaʻa ka cytopenia papa 3/4 i nā hihia āpau. ʻO ka lōʻihi o ka neutropenia he 7 mau lā. I ka lā 28, ʻike ʻia ka pae 3/4 neutropenia i 11/33 mau maʻi (33%) a ʻike ʻia ka pae 3/4 thrombocytopenia i 7/33 mau maʻi (21%). ʻO 1 wale nō ka mea maʻi (3%) i koi ʻia i ke komo ʻana i ka hale mālama koʻikoʻi, 2 mau maʻi (6%) i koi i ke kākoʻo vasopressor, 18 mau maʻi (55%) i loaʻa i ka tolumab, me ka median o 1 (1-4) a me 5 mau maʻi (15%) i loaʻa nā glucocorticoids. ʻO ka lōʻihi o ka noho ʻana he 8 mau lā (7-19 lā).
Hōʻike kēia ʻikepili piha i ka NexCAR19 i ka hopena maikaʻi a me ka ʻōlelo palekana i nā maʻi maʻi maʻi B-cell. ʻAʻohe ona ICANS, ʻoi aku ka pōkole o ka cytopenia, a me ka haʻahaʻa haʻahaʻa o ka papa 3/4 CRS, e lilo ia i hoʻokahi o nā huahana CD19 CAR T cell therapy palekana. Kōkua ka lāʻau lapaʻau e hoʻomaikaʻi i ka maʻalahi o ka hoʻohana ʻana i ka lāʻau cell T CAR i nā maʻi like ʻole.
Ma ASH 2023, ua hōʻike kekahi mea kākau e pili ana i ka hoʻohana ʻana i nā kumuwaiwai olakino i ka hoʻāʻo ʻana o ka pae 1/2 a me nā kumukūʻai e pili ana i ka mālama ʻana iā NexCAR19. ʻO ke kumu kūʻai o NexCAR19 i manaʻo ʻia ma 300 mau mea maʻi i kēlā me kēia makahiki ma kahi kumu hoʻohālike i hoʻopuehu ʻia ma kahi o $15,000 i kēlā me kēia maʻi. Ma kahi halemai hoʻonaʻauao, ʻo ke kumukūʻai maʻamau o ka hoʻokele lapaʻau (a hiki i ka hope hope) no ka mea maʻi ma kahi o $4,400 (ma kahi o $4,000 no lymphoma a me $5,565 no B-ALL). Ma kahi o 14 pākēneka wale nō o kēia mau koina no ka noho halemai.
Ka manawa kau: Apr-07-2024




