He 80%-85% o ka huina o na ma'i ma'i ma'i ma'i 'a'ole li'ili'i (NSCLC), a 'o ka 'oki 'oki 'ana ka mea 'oi loa no ka mālama 'ana i ka NSCLC. Eia naʻe, me ka 15% ho'ēmi i ka ho'i hou 'ana a me ka ho'omaika'i 'ana he 5% i ke ola 'ana o 5 makahiki ma hope o ka chemotherapy perioperative, aia ka nui o ka pono ma'i 'ole.
ʻO ka maʻi immunotherapy no ka NSCLC he wahi wela noiʻi hou i nā makahiki i hala iho nei, a ua hoʻokumu nā hopena o kahi helu 3 o nā hoʻokolohua hoʻokele randomized i ke kūlana koʻikoʻi o ka immunotherapy perioperative.
ʻO ka Immunotherapy no nā poʻe maʻi me ka operable early stage non-small cell lung cancer (NSCLC) ua holomua nui i nā makahiki i hala iho nei, a ʻo kēia hoʻolālā lapaʻau ʻaʻole e hoʻolōʻihi i ke ola o nā mea maʻi, akā e hoʻomaikaʻi pū i ka maikaʻi o ke ola, e hāʻawi ana i kahi hoʻohui kūpono i ka hana kuʻuna.
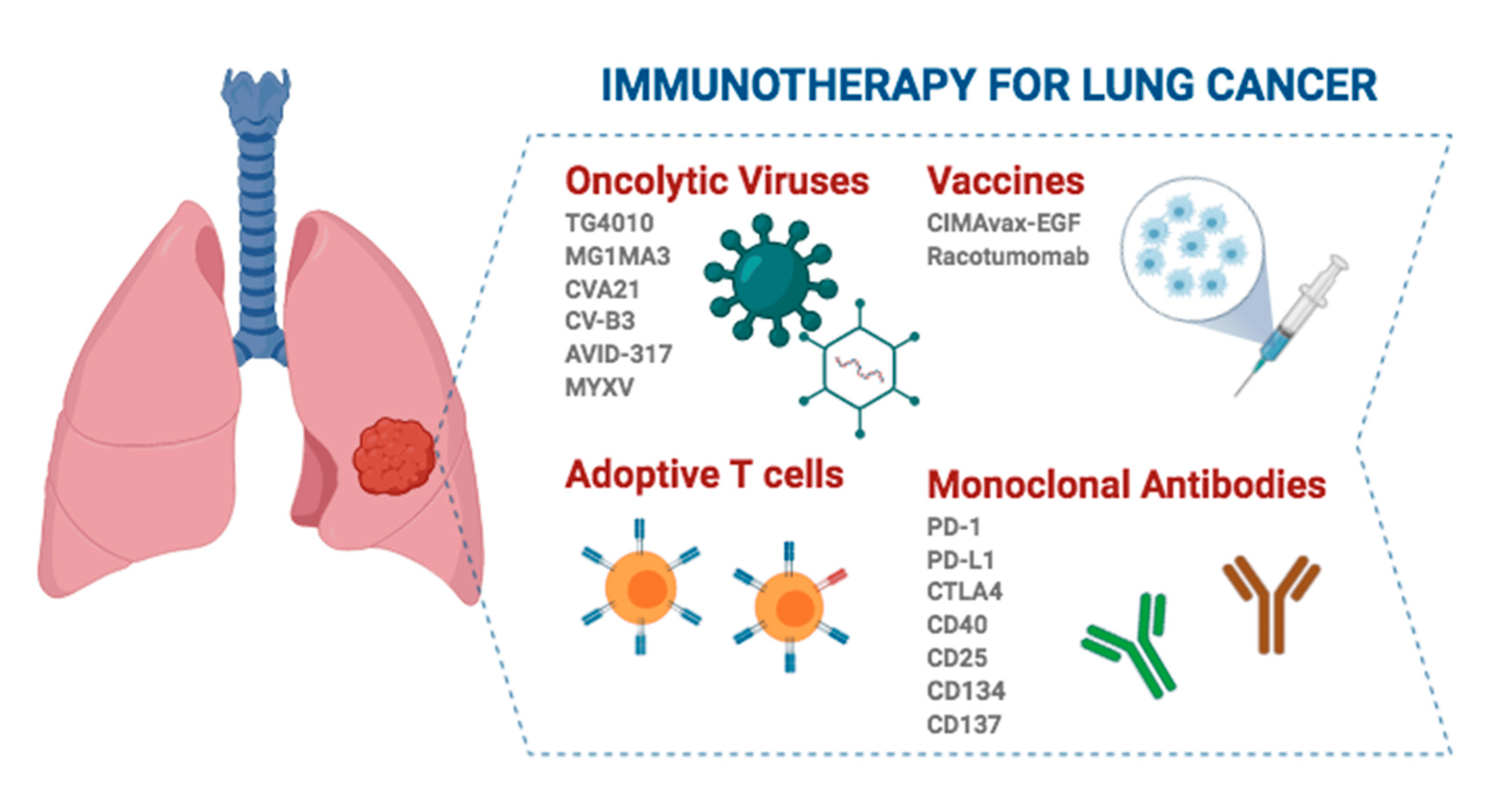
Ma muli o ka manawa e lawelawe ʻia ai ka immunotherapy, ʻekolu mau ʻano nui o ka immunotherapy i ka mālama ʻana i ka NSCLC hoʻomaka mua.
1. Neoadjuvant immunotherapy wale nō: Hana ʻia ka Immunotherapy ma mua o ka ʻoki ʻana e hoʻemi i ka nui o ka ʻōpū a hoʻemi i ka pilikia o ka hoʻi hou ʻana. Ua hōʻike ka CheckMate 816 [1] i ka immunotherapy i hui pū ʻia me ka chemotherapy i hoʻomaikaʻi nui i ke ola ʻole hanana hanana (EFS) i ka pae neoadjuvant i hoʻohālikelike ʻia me ka chemotherapy wale nō. Eia kekahi, hiki i ka neoadjuvant immunotherapy ke hoʻemi i ka helu hoʻihoʻi ʻana i ka wā e hoʻomaikaʻi ai i ka pathological complete response rate (pCR) o nā maʻi, a laila e hōʻemi ana i ka hiki ke hoʻi hou i ka postoperative.
2. Immunotherapy perioperative (neoadjuvant + adjuvant): Ma kēia ʻano, hoʻohana ʻia ka immunotherapy ma mua a ma hope o ke ʻoki ʻana e hoʻonui i kona hopena antitumor a hoʻoneʻe hou i nā liʻiliʻi liʻiliʻi ma hope o ke kaʻina. ʻO ka pahuhopu nui o kēia kŘkohu lapaʻau ʻo ia ka hoʻomaikaʻi ʻana i ke ola lōʻihi a me ka hoʻōla ʻana o nā maʻi maʻi maʻi ma o ka hoʻohui ʻana i ka immunotherapy ma nā pae neoadjuvant (pre-operative) a me ka adjuvant (post-operative). ʻO Keykeynote 671 kahi ʻelele o kēia kumu hoʻohālike [2]. ʻOiai ʻo ia wale nō ka randomized controlled trial (RCT) me ka maikaʻi EFS a me ka OS endpoints, ua loiloi ʻo ia i ka pono o ka palizumab i hui pū ʻia me ka chemotherapy i nā maʻi maʻi perioperatively resectable Ⅱ, ⅢA, a me ⅢB (N2) NSCLC. Ke hoʻohālikelikeʻia me ka chemotherapy wale nō,ʻo ka pembrolizumab i hui pūʻia me ka chemotherapy i hoʻonui i ka EFS ma waena o 2.5 mau makahiki a ho'ēmi i ka hopena o ka piʻiʻana o ka maʻi, hoʻi hou, a make paha e 41%; ʻO KEYNOTE-671 ka noiʻi immunotherapy mua loa e hōʻike i ka pōmaikaʻi ola holoʻokoʻa (OS) i ka NSCLC hiki ke hoʻihoʻi ʻia, me ka hōʻemi ʻana o 28% i ka hopena o ka make (HR, 0.72), kahi milestone i neoadjuvant a me adjuvant immunotherapy no ka NSCLC hoʻomaka mua.
3. Adjuvant immunotherapy wale nō: Ma kēia ʻano, ʻaʻole i loaʻa i ka poʻe maʻi ka lāʻau lapaʻau ma mua o ka ʻoki ʻana, a ua hoʻohana ʻia nā immunodrugs ma hope o ka ʻoki ʻana e pale ai i ka hoʻi hou ʻana o nā maʻi maʻi ʻokoʻa, kahi kūpono no nā poʻe maʻi me ka nui o ka hopena. Ua loiloi ka haʻawina IMpower010 i ka maikaʻi o ka postoperative adjuvant attilizumab versus optimal supportive therapy i nā poʻe maʻi me ka pae IB i hoʻopau loa ʻia i IIIA (AJCC 7th edition) NSCLC [3]. Ua hōʻike nā hopena i ka hoʻolōʻihi ʻana o ka lāʻau hoʻohui me ka attilizumab i ke ola ʻole o ka maʻi (DFS) i nā maʻi PD-L1 maikaʻi ma ka pae ⅱa i ⅢA. Eia kekahi, ua loiloi ka noiʻi KEYNOTE-091/PEARLS i ka hopena o ka pembrolizumab ma ke ʻano he adjunctive therapy i nā poʻe maʻi i hoʻopau ʻia me ka pae IB a i IIIA NSCLC [4]. Ua hoʻolōʻihi ʻia ʻo Pabolizumab i ka heluna kanaka holoʻokoʻa (HR, 0.76), me kahi DFS median o 53.6 mau mahina ma ka hui Pabolizumab a me 42 mau mahina ma ka hui placebo. I loko o ka subgroup o nā maʻi me PD-L1 tumor proportion score (TPS) ≥50%, ʻoiai ua hoʻolōʻihi ʻia ka DFS ma ka hui Pabolizumab, ʻaʻole nui ka ʻokoʻa ma waena o nā pūʻulu ʻelua ma muli o ka liʻiliʻi liʻiliʻi liʻiliʻi, a ʻoi aku ka lōʻihi o ka hahai ʻana e pono e hōʻoia.
Ma muli o ka hui ʻana o ka immunotherapy me nā lāʻau lapaʻau ʻē aʻe a i ʻole nā mea therapeutic a me ke ʻano hui pū, hiki ke hoʻokaʻawale ʻia ka papahana o neoadjuvant immunotherapy a me adjuvant immunotherapy i ʻekolu mau ʻano nui:
1. Hoʻokahi immunotherapy: Aia kēia ʻano lapaʻau i nā haʻawina e like me LCMC3 [5], IMpower010 [3], KEYNOTE-091/PEARLS [4], BR.31 [6], a me ANVIL [7], i hōʻike ʻia e ka hoʻohana ʻana i nā lāʻau immunotherapy hoʻokahi e like me ka adjuvant therapy.
2. ʻO ka hui pū ʻana o ka immunotherapy a me ka chemotherapy: ʻO ia mau haʻawina ʻo KEYNOTE-671 [2], CheckMate 77T [8], AEGEAN [9], RATIONALE-315 [10], Neotorch [11], a me IMpower030 [12]. Ua nānā kēia mau haʻawina i nā hopena o ka hoʻohui ʻana i ka immunotherapy a me ka chemotherapy i ka wā perioperative.
3. Ka hui 'ana o ka immunotherapy me nā 'ano lā'au lapa'au 'ē a'e: (1) Hui pū me nā immunodrugs 'ē a'e: No ka la'ana, ua hui 'ia ka antigen 4 (CTLA-4) cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) ma NEOSTAR ho'ā'o [13], lymphocyte activation gene 3 (LAG-3) antibody i hui 'ia ma NEO-Predict4 a me ka Imunolobulin (1TIM) cell. ua hui pū ʻia nā hale i ka hoʻāʻo ʻo SKYSCRAPER 15 ʻO nā haʻawina e like me TIGIT antibody hui [15] ua hoʻonui i ka hopena anti-tumor ma o ka hui ʻana o nā lāʻau lapaʻau. (2) Hoʻohuiʻia me ka radiotherapy: no ka laʻana, ua hoʻolālāʻia ka duvaliumab i hui pūʻia me ka radiotherapy stereotactic (SBRT) e hoʻonui i ka hopena o ka NSCLC mua [16]; (3) Hui pū me nā lāʻau anti-angiogenic: No ka laʻana, ua ʻimi ka EAST ENERGY [17] i ka hopena synergistic o ka ramumab i hui pū ʻia me ka immunotherapy. Hōʻike ka ʻimi ʻana i nā ʻano immunotherapy he nui ʻaʻole maopopo loa ke ʻano o ka noi ʻana o ka immunotherapy i ka wā perioperative. ʻOiai ʻo ka immunotherapy wale nō i hōʻike i nā hopena maikaʻi i ka lāʻau perioperative, ma ka hui pū ʻana i ka chemotherapy, radiation therapy, antiangiogenic therapy, a me nā mea pale ʻē aʻe e like me CTLA-4, LAG-3, a me TIGIT, manaʻo nā mea noiʻi e hoʻonui hou i ka pono o ka immunotherapy.
ʻAʻohe manaʻo hoʻoholo no ke ʻano maikaʻi loa o ka immunotherapy no ka NSCLC mua o ka hoʻohana ʻana, ʻoi aku ka nui o ka immunotherapy perioperative i hoʻohālikelike ʻia me ka immunotherapy neoadjuvant wale nō, a inā hiki i ka immunotherapy adjuvant hou ke lawe mai i nā hopena hou aʻe, aia nō ka nele o nā hopena hoʻohālikelike pololei.
Forde et al. Ua hoʻohana i ka exploratory propensity score weighted analysis e hoʻohālikelike i ka hopena o nā hoʻokolohua i hoʻopaʻa ʻia, a hoʻoponopono i ka helu helu kumu a me nā ʻano maʻi ma waena o nā kānaka noiʻi like ʻole e hōʻemi i ka hopena confounding o kēia mau kumu, e hoʻohālikelike ai i nā hopena o CheckMate 816 [1] a me CheckMate 77T [8]. ʻO ka manawa ma waena o ka hahai ʻana he 29.5 mau mahina (CheckMate 816) a me 33.3 mau mahina (CheckMate 77T), i kēlā me kēia, e hāʻawi ana i ka manawa hahai e nānā i ka EFS a me nā ʻano hana ʻē aʻe.
Ma ka loiloi paona, ʻo ka HR o EFS he 0.61 (95% CI, 0.39 a 0.97), e hōʻike ana i ka 39% haʻahaʻa haʻahaʻa o ka hoʻi hou ʻana a i ʻole ka make i ka perioperative nabuliumab hui pūʻulu chemotherapy (CheckMate 77T mode) i hoʻohālikelike ʻia me ka neoadjuvant nabuliumab hui pūʻulu chemotherapy (CheckMate 816). Ua hōʻike ka perioperative nebuliuzumab me ka hui chemotherapy i ka pōmaikaʻi haʻahaʻa i nā poʻe maʻi āpau i ka pae baseline, a ua ʻoi aku ka manaʻo o ka hopena i nā maʻi me ka liʻiliʻi o ka 1% tumor PD-L1 hōʻike (49% hoʻemi i ka hopena o ka hoʻi hou ʻana a i ʻole ka make). Eia hou, no ka poʻe maʻi i hikiʻole ke hoʻokō i ka pCR, ua hōʻike ka perioperative nabuliumab hui chemotherapy i ka pōmaikaʻi nui aʻe o ka EFS (35% ka ho'ēmiʻana i ka hopena o ka hoʻi hou a iʻole ka make) ma mua o ka neoadjuvant nabuliumab hui pūʻulu chemotherapy. Hōʻike kēia mau hopena e ʻoi aku ka maikaʻi o ke kumu hoʻohālike immunotherapy perioperative ma mua o ke kumu hoʻohālike neoadjuvant immunotherapy wale nō, ʻoi aku ka nui o nā maʻi me ka haʻahaʻa haʻahaʻa PD-L1 a me nā koena tumo ma hope o ka mālama mua ʻana.
Eia naʻe, ʻaʻohe ʻokoʻa koʻikoʻi o ke ola ʻana ma waena o neoadjuvant immunotherapy a me perioperative immunotherapy [18]. Ua ʻike ʻia kahi meta-analysis e pili ana i ka ʻikepili maʻi hoʻokahi i loaʻa nā hopena like ʻole o ka immunotherapy perioperative a me ka immunotherapy neoadjuvant ma EFS ma nā puʻupuʻu pCR a me non-PCR i nā maʻi me ka NSCLC hoʻomaka mua [19]. Eia kekahi, ʻo ka hāʻawi ʻana o ka adjuvant immunotherapy phase, ʻoi aku ka nui ma hope o ka loaʻa ʻana o ka pCR i nā mea maʻi, he wahi hoʻopaʻapaʻa i loko o ke keʻena.
I kēia mau lā, ua kūkākūkā ka US Food and Drug Administration (FDA) Oncology Drugs Advisory Committee i kēia pilikia, me ka ʻōlelo ʻana ʻaʻole maopopo ka hana kūikawā o ka immunotherapy adjuvant [20]. Ua kūkākūkā ʻia: (1) He paʻakikī ke hoʻokaʻawale i nā hopena o kēlā me kēia pae o ka lāʻau lapaʻau: no ka mea, ʻo ka papahana perioperative he ʻelua mau māhele, neoadjuvant a me adjuvant, he paʻakikī ke hoʻoholo i ka hāʻawi ʻana o kēlā me kēia māhele i ka hopena holoʻokoʻa, e paʻakikī ai ka hoʻoholo ʻana i ka manawa i ʻoi aku ka koʻikoʻi, a i ʻole pono e hana ʻia nā ʻāpana ʻelua i ka manawa like; (2) ʻO ka hiki ke hoʻomaʻamaʻa nui: inā pili ka immunotherapy i nā ʻāpana lapaʻau ʻelua, hiki i nā mea maʻi ke loaʻa ka hoʻomaʻamaʻa nui a hoʻonui i ka hopena o nā hopena ʻaoʻao; (3) Hoʻonui ʻia ke kaumaha o ka mālama ʻana: ʻO ka mālama hou ʻana i ka pae lapaʻau adjuvant hiki ke alakaʻi i ke kaumaha o ka mālama ʻana no nā maʻi, ʻoi aku ka maopopo ʻole o kāna hāʻawi ʻana i ka pono holoʻokoʻa. I ka pane ʻana i ka hoʻopaʻapaʻa ma luna, i mea e huki ai i kahi hopena maopopo, pono nā hoʻokolohua hoʻokele randomized i hoʻolālā ʻia no ka hōʻoia hou ʻana i ka wā e hiki mai ana.
Ka manawa hoʻouna: Dec-07-2024