I kēia mau lā, ua lilo ka maʻi ʻaʻai momona Nonalcoholic (NAFLD) i kumu nui o ka maʻi ate maʻi ma Kina a me ka honua. Aia ka ma'i ma'i hepatic steatohepatitis ma'alahi, nonalcoholic steatohepatitis (NASH) a me ka cirrhosis pili a me ka ma'i 'a'ai. Hōʻike ʻia ka NASH e ka hoʻonui ʻana i ka momona i loko o nā hepatocytes a hoʻoulu ʻia i ka pōʻino cellular a me ka mumū, me ka hepatic fibrosis a i ʻole. ʻO ke koʻikoʻi o ka fibrosis ate i nā poʻe maʻi NASH e pili kokoke ana me ka prognosis ate ʻino (cirrhosis a me kona mau pilikia a me ka maʻi hepatocellular carcinoma), nā hanana cardiovascular, extrahepatic malignancies, a me nā kumu āpau. Hiki i ka NASH ke hoʻopilikia i ka maikaʻi o ke ola o nā maʻi; akā naʻe, ʻaʻohe lāʻau lapaʻau a lapaʻau paha i ʻae ʻia e mālama iā NASH.
ʻO kahi noiʻi hou (ENLIVEN) i paʻi ʻia ma ka New England Journal of Medicine (NEJM) i hōʻike i ka pegozafermin i hoʻomaikaʻi i ka fibrosis ate a me ka mumū o ka ate i nā maʻi NASH non-cirrhotic i hōʻoia ʻia i ka biopsy.
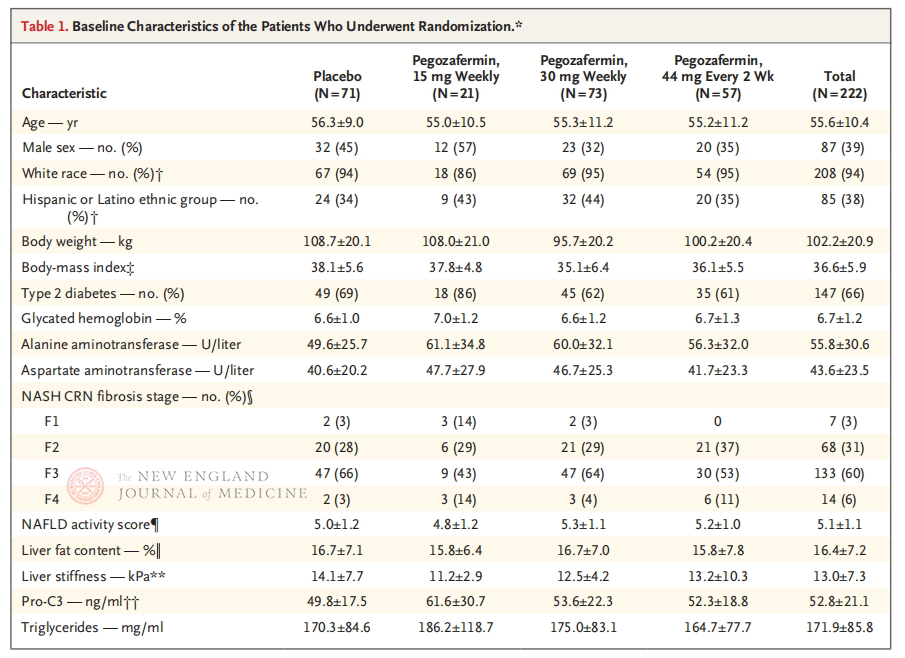
ʻO ka multicenter, randomized, double-blind, placebo-controlled Phase 2b clinical trial, i alakaʻi ʻia e Professor Rohit Loomba a me kāna hui lapaʻau ma ke Kulanui o Kaleponi, San Diego School of Medicine, ua hoʻopaʻa inoa i nā maʻi 222 me ka pae biopsie-hōʻoia F2-3 NASH ma waena o Kepakemapa 28, 2021 a me ʻAukake 15, 2022. 15 mg a i ʻole 30 mg i hoʻokahi pule, a i ʻole 44 mg hoʻokahi i kēlā me kēia 2 pule) a i ʻole placebo (hoʻokahi manawa i ka pule a i ʻole hoʻokahi i kēlā me kēia 2 pule). Loaʻa nā helu hope mua i ka ≥ pae 1 hoʻomaikaʻi i ka fibrosis a ʻaʻohe holomua o NASH. Hoʻoholo ʻia ʻo NASH me ka ʻole o ka holomua fibrotic. Ua hana pū ka haʻawina i kahi loiloi palekana.
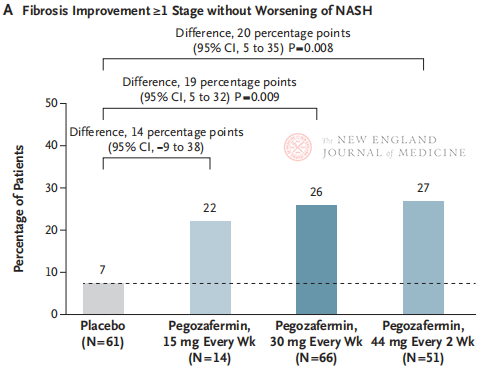
Ma hope o 24 mau pule o ka mālama ʻana, ʻoi aku ka kiʻekiʻe o ka nui o nā mea maʻi me ≥ pae 1 i ka fibrosis a ʻaʻole i hōʻino ʻia ka NASH, a ʻo ka hapa o nā mea maʻi me ka regression o NASH a me ka ʻole o ka piʻi ʻana o ka fibrosis i ʻoi aku ka kiʻekiʻe ma nā hui ʻekolu Pegozafermin ma mua o ka hui placebo, me ka nui o nā ʻokoʻa nui i nā maʻi i mālama ʻia me 44 mg 30 mg hoʻokahi i kēlā me kēia pule. Ma ke ʻano o ka palekana, ua like ka pegozafermin me ka placebo. ʻO nā hopena ʻino loa e pili ana i ka mālama ʻana i ka pegozafermin, ʻo ia ka nausea, ka maʻi maʻi, a me ka erythema ma ka wahi i hoʻopaʻa ʻia. Ma kēia hoʻokolohua 2b, hōʻike nā hopena mua e hoʻomaikaʻi ka mālama ʻana me ka pegozafermin i ka fibrosis ate.
ʻO pegozafermin, i hoʻohana ʻia i kēia haʻawina, he analogue glycolated lōʻihi o ke kanaka fibroblast growth factor 21 (FGF21). ʻO FGF21 he hormone metabolic endogenous i huna ʻia e ke ake, kahi i hana i ka hoʻoponopono ʻana i ka lipid a me ka metabolism glucose. Ua hōʻike mua nā haʻawina i ka FGF21 i nā hopena therapeutic i nā poʻe maʻi NASH ma o ka hoʻonui ʻana i ka naʻau o ka insulin o ka ate, hoʻoulu ʻana i ka momona momona, a me ka pale ʻana i ka lipogenesis. Eia nō naʻe, ʻo ka hapalua o ke ola o ka FGF21 maoli (e pili ana i 2 mau hola) e kaupalena i kona hoʻohana ʻana i ka lāʻau lapaʻau o NASH. Hoʻohana ka pegozafermin i ka ʻenehana pegylation glycosylated e hoʻolōʻihi i ka hapalua o ke ola o ka FGF21 maoli a hoʻonui i kāna hana olaola.
Ma waho aʻe o nā hopena maikaʻi ma kēia hoʻokolohua lapaʻau Phase 2b, ua hōʻike ʻia kahi noiʻi hou i paʻi ʻia ma Nature Medicine (ENTRIGUE) ua hōʻemi nui ka pegozafermin i nā triglycerides, non-HDL cholesterol, apolipoprotein B, a me ka steatosis hepatic i nā maʻi me ka hypertriglyceridemia koʻikoʻi.
Ke hōʻike nei kēia mau haʻawina e hiki i ka pegozafermin, ma ke ʻano he hormone metabolic endogenous, ke hāʻawi i nā pōmaikaʻi metabolic he nui i nā poʻe maʻi me NASH, ʻoi aku hoʻi no ka mea hiki ke kapa hou ʻia ʻo NASH i ka maʻi momona momona pili i ka metabolic i ka wā e hiki mai ana. ʻO kēia mau hopena e lilo ia i lāʻau lapaʻau koʻikoʻi loa no ka mālama ʻana iā NASH. Ma ka manawa like, e kākoʻo kēia mau hopena haʻawina maikaʻi i ka pegozafermin i loko o nā hoʻokolohua lapaʻau 3.
ʻOiai ua loaʻa i ka lāʻau pegozafermin biweekly 44 mg a i ʻole 30 mg i kēlā me kēia pule ka histological kumu mua o ka hoʻokolokolo, ʻo ka lōʻihi o ka mālama ʻana i kēia noiʻi he 24 mau pule wale nō, a ʻo ka helu hoʻokō i ka hui placebo he 7% wale nō, ʻoi aku ka haʻahaʻa ma mua o nā hopena o nā noiʻi ma mua o 48 mau pule. Ua like nā ʻokoʻa a me ka palekana? Hāʻawi ʻia i ka heterogeneity o NASH, ʻoi aku ka nui, multi-center, pono nā hoʻokolohua lapaʻau honua i ka wā e hiki mai ana e hoʻokomo i ka nui o ka poʻe maʻi a hoʻonui i ka lōʻihi o ka mālama ʻana no ka loiloi maikaʻi ʻana i ka pono a me ka palekana o ka lāʻau.
Ka manawa hoʻouna: Sep-16-2023